임상약물유전체 연구부
연구영역
임상약물유전체학 (Clinical Pharmacogenomics)
| 주요연구분야 | Tailored pharmacotherapy |
|---|---|
| Pharmacokinetic and pharmacodynamic study in relation to pharmacogenetics | |
| Pharmacogenetic epidemiology | |
| Pharmacogenetics in the drug discovery and development |
약물분석/대사체학
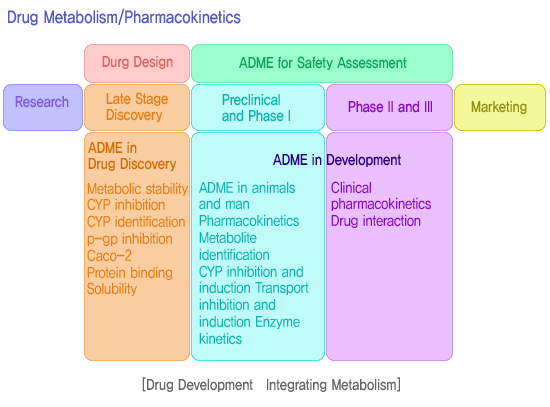
| 주요연구분야 | Drug Metabolism/Pharmacokinetics |
|---|---|
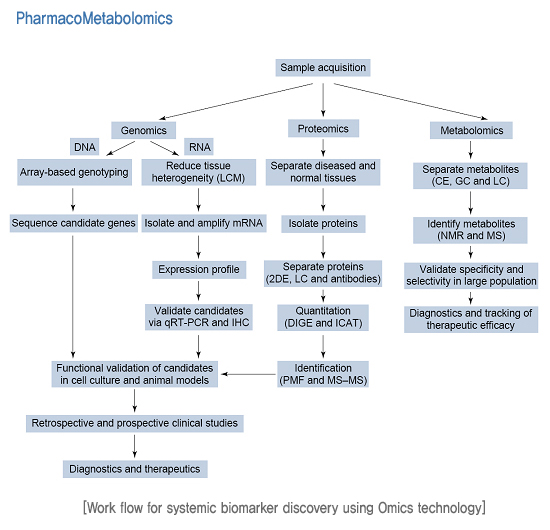
| PharmacoMetabolomics | |
| Bioanalysis |
DMPK Key Technology
| 항목 | 요소기술 | |
|---|---|---|
| In vitro metabolism | Metabolic stability | Metabolic stability in microsomes, hepatocytes, plasma, blood |
| Subcellular metabolism | Hepatocytes, S9 fraction, cytosol, microsomes, intestine | |
| Metabolic route and kinetics | Reaction phenotyping and kinetics of Phase I (CYPs) and Phase II (UGT) enzymes | |
| Interspecies comparative metabolism | Monkey, dog, hamster, mouse, rabbit, rat | |
| Metabolite Identification | Metabolite structure ID, toxic metabolite ID | |
| In vitro transport | Permeability test |
Permeability in Caco-2, MDCK, LLC-PK1 cells Cryopreserved hepatocytes uptake Biliary excretion in sandwich cultured hepatocytes |
| Transport system and kinetics |
Uptake screening test in Xenopus oocytes Efflux screening test in MDCK-MDR1, MRPs, BCRP |
|
| Pharmacogenetics study | Transport activity in wild type and variant transporters | |
| Drug-drug interaction | Inhibition |
Screening of inhibitory potential of CYPs, UGTs, transporters Estimation of IC50 (Ki) value |
| Mechanism-based inhibition |
IC50 shift assay, reversibility assay Estimation of KI and kinact value |
|
| Induction |
Reporter assay mRNA expression level in hepatocytes Phenotyping assay using specific substrates |
|
| In vivo PK (preclinical) |
Mass balance | Mass balance study using radioactive or non-radioactive compounds |
| Absorption | Estimation of bioavailability | |
| Distribution |
Tissue distribution (brain, liver, kidney etc) Blood partitioning, Protein binding |
|
| Metabolic profiling | Metabolites profiling and Met ID | |
| Excretion | Renal excretion, biliary excretion, enterohepatic circulation | |
| In vivo PK (human) |
Translational Research | In vitro to in vivo extrapolation (SimCYP) |
| Interspecies Pharmacokinetic Scaling | ||
| Consultation of decision making for further clinical development | ||
| Phase 0 study | Microdosing study | |
| Phase I study | First in human study | |
| Absolute bioavailability | ||
| Drug-drug interaction potential study (cocktail study) | ||
| Genotype based ADME study | ||
| Special population (Gender, nephropathy, hepatic failure, Elderly) | ||
| Bridging study |